Regulations
FDA Proposes Front-of-Package Label Rule For Saturated Fat, Sodium, Added Sugar Content at a Glance
The new rule aims to make it easier for consumers to see if a product has “low,” “medium,” or “high” concentrations of these nutrients.

By: Mike Montemarano
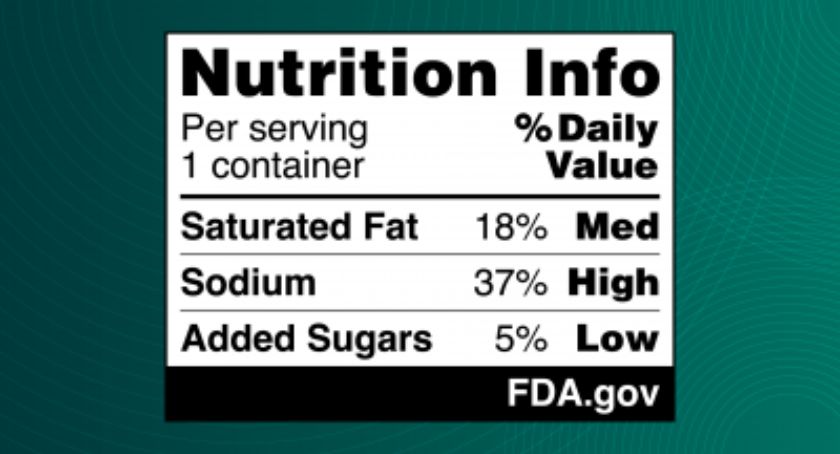
The U.S. Food and Drug Administration (FDA) is proposing a new rule that would require a front-of-package nutrition labeling scheme to highlight the saturated fat, sodium, and added sugar content of food products so consumers can more quickly and easily identify healthier food choices.
“The U.S. faces an ever-growing epidemic of preventable diet-related chronic diseases such as cardiovascular disease, diabetes, and obesity,” the agency stated. “Improving nutrition offers one of the greatest opportunities for reducing these and other chronic illnesses and premature death.”
The FDA’s proposed front-of-packaging nutrition label, referred to as a Nutrition Info box, would complement the Nutrition Facts label. It would display simplified information that interprets the saturated fat, sodium, and added sugar content of a food as “low,” “med,” or “high” on the front of food packages. The agency noted that federal dietary recommendations are to limit these three nutrients to achieve a nutrient-dense diet within calorie limits. A manufacturer could also voluntarily declare calories in the proposed Nutrition Info box, per existing FDA regulations.
By having this information on the front of packages, consumers could compare similar foods and identify foods with healthier nutrient profiles based on this information, such as by quickly spotting one yogurt that is lower in added sugar, for instance.
Front-of-packaging nutrition labeling has increased dramatically around the world in recent years, FDA noted, and this “has the potential to be a landmark policy and as iconic as the Nutrition Facts label,” the agency said.




















