Features
Detecting Adulteration: Huperzine A Case Study
Carbon-14 testing offers a reliable means to differentiate between natural-derived and petrochemical-derived sources.

By: Haley Gershon
Marketing Manager, Beta Analytic

The dietary supplement industry is prone to economically motivated adulteration due to increasing popularity of natural-sourced ingredients. Huperzine A, a supplement ingredient extracted from the Chinese club moss Huperzia serrata, is an example of a plant-based nutraceutical that is vulnerable to adulteration through the fraudulent use of petrochemical-derived synthetics. Within the market, in some cases, Huperzine A supplements that have been exposed to ingredient adulteration are still claimed as natural-sourced on product labels.1-2 As a result, the supplement industry depends on analytical techniques to detect
ingredient adulteration.
Choosing a robust quality control method, however, is a common challenge for manufacturers. This article focuses on verification of the source of three different brands of Huperzine A plant extract samples through the comparison of two different methods: high-performance liquid chromatography (HPLC) fingerprinting, and carbon-14 (radiocarbon) analysis.
Huperzine Adulteration
Huperzine A is a widely-used ingredient in the nutraceuticals industry for a variety of health purposes, such as memory enhancement.3 The global Huperzine A market size is forecasted to expand at a compound annual growth rate of 6.68% from 2019 to 2024.4 This growth coincides with increasing demand for Huperzine A by research and development teams during product development as a result of its potential in treating diseases like Alzheimer’s.5,6
Due to the expensive cost of natural Huperzine A, the plant extract is vulnerable to ingredient adulteration. For example, purified (99%) Huperzine A can sell for more than $700 per gram, while the petrochemical-derived synthetic version is cheaper, potentially leading manufacturers and distributors to substitute the high-priced natural version for the adulterated ingredient.7,8 In order to detect adulteration of Huperzine A, quality control departments often submit samples to undergo analyses for ingredient verification.
Comparison of HPLC & Carbon-14
There are several analytical methods available for detecting ingredient adulteration. This includes HPLC and carbon-14 analysis, however, the two methods differ vastly in the type of adulteration they can detect.
HPLC is a quality control technique used to separate, identify, and quantify different components in a mixture. It is also able to determine the purity of a compound.9 When analyzing a sample using HPLC, a mixture or a single compound is passed in a solution through a stationary phase at high pressure, which separates the components from the stationary phase based on different polarities of the compounds.10 Although HPLC-fingerprinting is useful in identifying the source of a plant extract, it has several limitations when it comes to detecting adulterants. For example, the analysis detects the addition of a synthetic adulterant, however, it is unable to identify the substitution of a plant-based ingredient with a petrochemical-derived adulterant.11-12
Carbon-14 testing can differentiate between plant-based and petroleum-derived ingredients, and therefore can confirm the presence of petrochemical-derived synthetics. The analysis is based on the ASTM D6866 standard, which determines if the components in a material are sourced from biological by-products.13 The analysis uses an accelerator mass spectrometer instrument, which counts the amount of carbon-14 content present in a given sample. The portion of carbon-14 present is representative of the percentage of biomass-derived sources. Therefore, carbon-14 testing can authenticate naturally sourced ingredients while detecting petrochemical adulterants present. Carbon-14 analysis of plant extract ingredients yields results that can range from 0% bio-based to 100% bio-based. Samples with the latter result are fully composed of natural-derived sources.14
Case Study
A case study of three different brands of Huperzine A supplements were subject to both HPLC fingerprinting, performed at Nanjing Hup Chemical laboratory, and carbon-14 testing, performed by Beta Analytic. The three products were all labeled as natural-sourced Huperzine A plant extract. The purpose of the analyses was to expose the potential of plant extract adulteration within the nutraceutical industry.
HPLC-fingerprinting was first performed on all three samples, which demonstrated results confirming Huperzine A ingredients were derived from plant extracts. This, however, does not indicate that the ingredients were natural-derived plant extracts, but rather suggests there was no addition of synthetic ingredients.
Prior to performing carbon-14 testing on each supplement sample, the Huperzine A ingredient was first isolated from the samples and highly purified up to 99%, since the amount of Huperzine A present in each product was less than 1%.
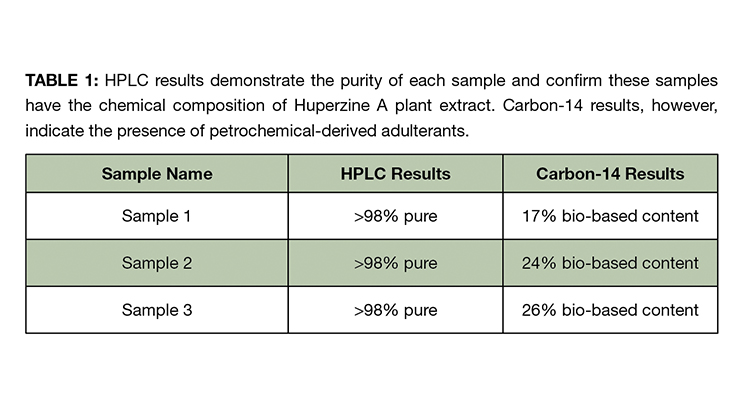
While results from HPLC fingerprinting demonstrated that the purity of Huperzine A in each supplement sample was more than 98% pure, the carbon-14 results confirmed the presence of ingredient adulteration in all three samples, demonstrating evidence of petrochemical-derived sources in the analyzed material. The results highlight the vulnerability of Huperzine A to adulteration, providing reason for quality control departments to confirm ingredient authentication via carbon-14 analysis. Table 1 summarizes the HPLC and carbon-14 results of the analyzed samples.
Conclusion
As demonstrated through this case study, applying rigorous methods of ingredient verification to detect adulteration is vital for quality control procedures. While HPLC can identify different compounds in a mixture and quantify the purity of the compounds, there are limitations concerning detection of substituting plant-based ingredients with petrochemical-derived adulterants.
Carbon-14 testing provides a means to differentiate between natural-derived and petrochemical-derived sources, yielding a result which contains the portion of plant-based, or bio-based, content. Employing carbon-14 analysis to Huperzine A is a key step for quality assurance and quality control in order to avoid adulteration of the nutraceutical ingredient in the marketplace.
References
- Ma, X. How to identify the source of Huperzine A (HupA). (Oct 2019). https://www.linkedin.com/pulse/how-identify-source-huperzine-hupa-xin-ma/
- Absorb Your Health. What Is Huperzine A Extract? A Review Of Benefits, Side Effects, And Dosage. (23 Dec 2017). https://www.absorbyourhealth.com/huperzine-extract-review-benefits-side-effects-dosage/
- Epilepsy Foundation. Interview with Dr. Steve Schachter on Investigational Therapy. (12 March 2019). https://www.epilepsy.com/article/2019/3/interview-dr-steve-schachter-investigational-therapy
- Gen Consulting Company. Global Huperzine A Market Outlook 2019-2024. Accessed: 23 April 2020
- Barden, D. Rapid route to huperzine A. Chemistry World. (25 August 2011). https://www.chemistryworld.com/news/rapid-route-to-huperzine-a-/3003393.article
- Ha, G.T., Wong, R.K. and Zhang, Y. Huperzine A as Potential Treatment of Alzheimer’s Disease: An Assessment on Chemistry, Pharmacology, and Clinical Studies. Chemistry & Biodiversity, 8: 1189-1204. (18 July 2011). doi:10.1002/cbdv.201000269
- Millipore Sigma. (±)-Huperzine A. Accessed 23 April 2020. https://www.sigmaaldrich.com/catalog/product/sigma/h5777?lang=en®ion=US
- Plant Use. Huperzia serrata (PROSEA). Accessed 23 April 2020. https://uses.plantnet-project.org/en/Huperzia_serrata_(PROSEA)
- ScienceDirect. High-Performance Liquid Chromatography. Accessed 23 April 2020. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/high-performance-liquid-chromatography
- Hello Bio. Understanding purity and quality – a guide for life scientists. Accessed 23 April 2020. https://www.hellobio.com/purity-guide-for-life-scientists/
- Wolfender, J-L. HPLC in Natural Product Analysis: The Detection Issue. (14 January 2009). https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0028-1088393
- Beta Analytic. 3 Types of Adulteration in Dietary Supplements and How to Detect Them. (21 December 2018). https://www.betalabservices.com/dietary-supplements-adulteration/
- ASTM International. ASTM D6866 – 20 Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. Accessed 23 April 2020. https://www.astm.org/Standards/D6866.htm
- Beta Analytic. Carbon-14 Analysis Enhances Quality Control for Natural Products. Accessed 23 April 2020. https://www.betalabservices.com/natural-products.html


